Overview of Catalytic Reformer Units:
Critical in industries such as oil refining, petrochemicals, and hydrogen production, catalytic reformer units (CRUs) are primarily used to convert low-octane naphtha into high-octane reformate – a valuable blending component for gasoline. The process also produces hydrogen, which is used in other refinery processes such as hydrocracking and hydrotreating.
Damage mechanism reviews (DMRs) in catalyst reformers typically focus on identifying causes of degradation or failure in the reformer unit. Damage to these reformers can result from multiple mechanisms, often leading to inefficiencies, unplanned shutdowns, or costly repairs.
Key Functions of a Catalytic Reformer Unit:
- Octane Improvement: The primary function of a CRU is to increase the octane number of naphtha feedstock. This is achieved through several chemical reactions, including:
- Dehydrogenation: Converts naphthenes (cyclic hydrocarbons) into aromatics, boosting octane levels.
- Isomerization: Rearranges linear paraffins into branched isomers, which have higher octane ratings.
- Hydrogen Production: The reforming process generates significant amounts of hydrogen as a byproduct. This hydrogen is essential for other refinery processes, such as hydrocracking and hydrotreating, which are used to remove sulfur and other impurities from various hydrocarbon streams.
- Chemical Reactions: The catalytic reforming process involves several key reactions:
- Dehydrogenation of naphthenes to aromatics reaction – endothermic and produces hydrogen
- Dehydrocyclization of paraffins to aromatics – produces hydrogen
- Isomerization of normal paraffins to isoparaffins that do not produce or consume hydrogen.
- Hydrocracking of paraffins to smaller molecules – consumes hydrogen
Types of Catalytic Reformer Units
CRUs are classified into three main types based on their operational and regeneration methods: semi-regenerative reformers, continuous catalyst regeneration (CCR) reformers, and cyclic reformers.
1. Semi-Regenerative Reformers: This is the original and simplest type of catalytic reformer. All reactors are in service during normal operation and are brought offline simultaneously for catalyst regeneration. They typically operate at higher pressures to minimize coke formation on the catalyst, which extends the run length between outages but reduces octane improvement and hydrogen production.
2. Continuous Catalyst Regeneration (CCR) Reformers: In this type, the catalyst is continuously circulated through the reactors and a regenerator vessel. CCR units can operate at lower pressures, optimizing octane improvement and increasing hydrogen yield. In addition, they have the longest runs between outages due to continuous catalyst regeneration.
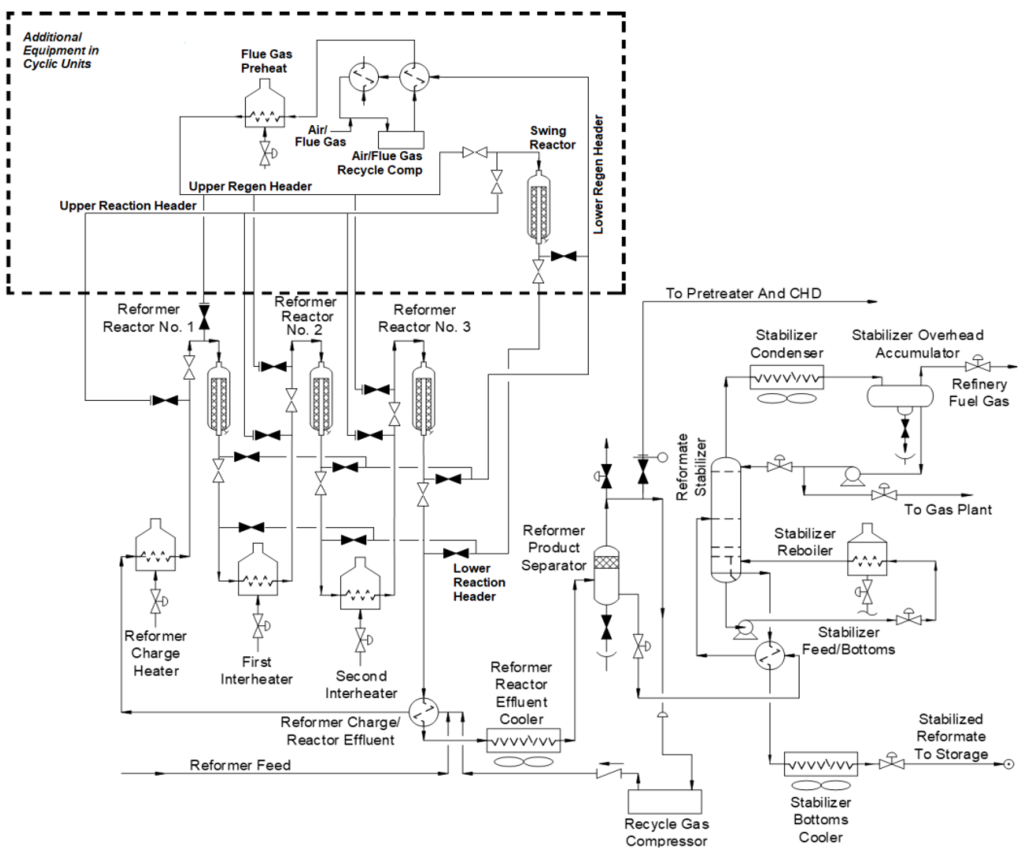
3. Cyclic Reformers: This is a hybrid version of semi-regenerative and CCR layouts. It has side-by-side reactors with a “swing” reactor that can be taken offline for regeneration while the other reactors remain in service.
Each type of reformer has its own advantages and is selected based on the specific needs and constraints of the refinery. Semi-regenerative reformers are simpler and less expensive but require more frequent shutdowns for regeneration. CCR reformers, while more complex and costly, offer continuous operation and higher efficiency. Cyclic reformers provide a middle ground, offering some of the benefits of continuous regeneration without the need for a fully continuous system.
Primary Pieces of Equipment in a Catalytic Reformer Unit
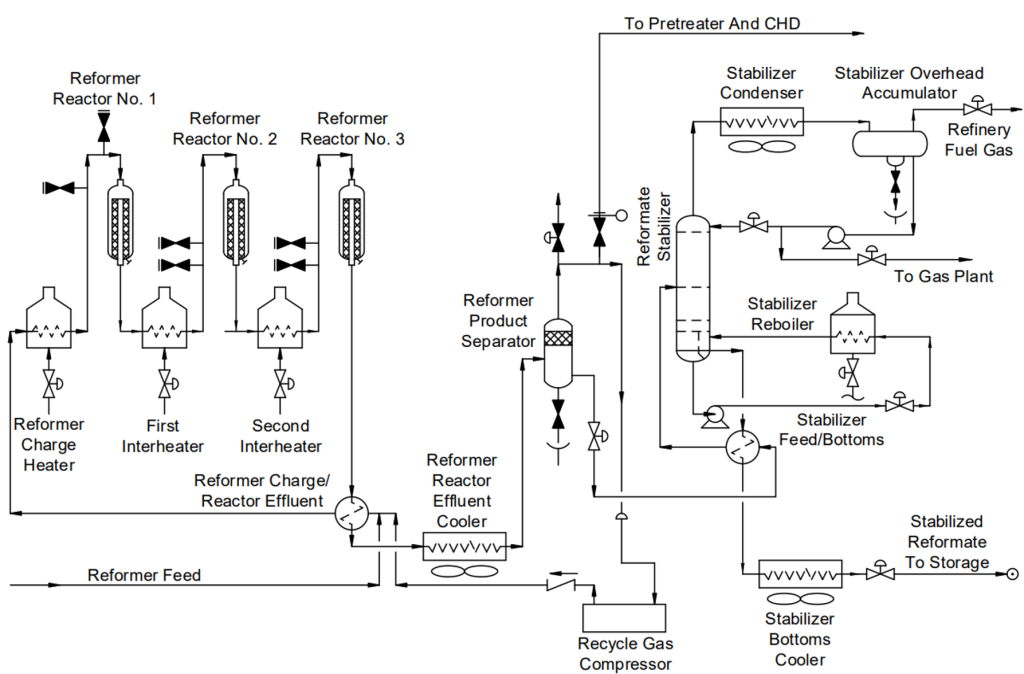
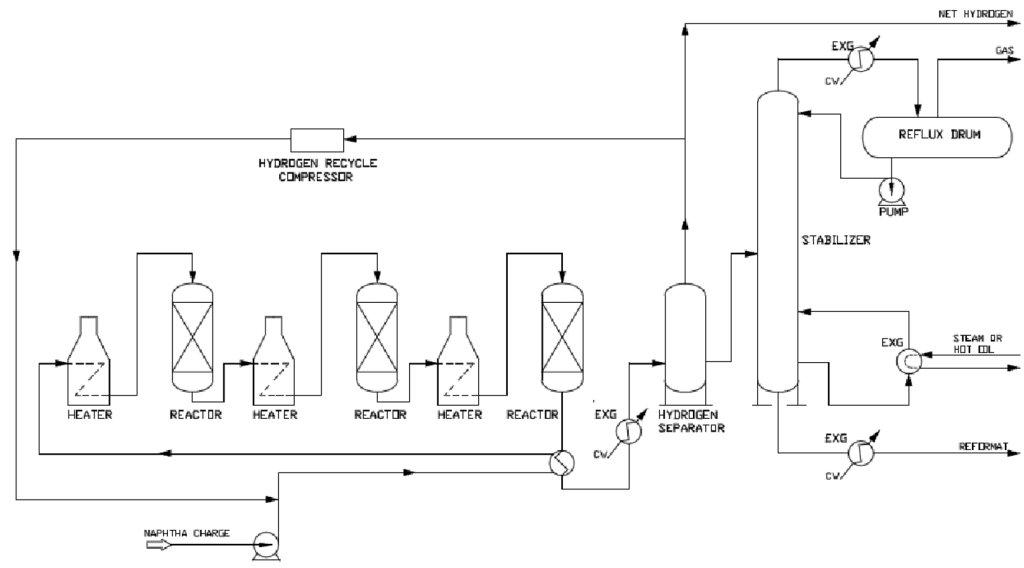
A CRU is a complex system comprising of several key pieces of equipment that are designed to convert low-octane naphtha into high-octane reformate. The primary pieces of equipment in a CRU are:
- Reactors: Typically, a CRU consists of three or more reactors arranged in series. These reactors facilitate the catalytic reactions necessary for reforming, such as dehydrogenation, dehydrocyclization, isomerization, and hydrocracking. Reactors are often constructed from Cr-Mo steels to resist high temperatures and hydrogen environments.
- Heaters: Fired heaters are used to preheat the feedstock before it enters the reactors. They provide the necessary heat to drive the endothermic reactions in the reactors. Heater tubes are typically made from high-temperature alloys like 9Cr-1Mo to resist oxidation and carburization.
- Heat Exchangers: Various heat exchangers (HE) are used to recover heat from the reactor effluent and preheat the feedstock. HEs improve the thermal efficiency of the unit by transferring heat from the hot reactor effluent to the incoming feed. Typical construction materials are carbon steel and stainless steel, depending on the specific service and operating conditions.
- Hydrogen Separator: A separator is used to separate hydrogen from the reactor effluent. The separated hydrogen is recycled back into the reactors to maintain the hydrogen partial pressure necessary for the reforming reactions. Materials of construction should be resistant to hydrogen embrittlement, such as low-alloy steels.
- Stabilizer Column: Distillation column is used to stabilize the reformate product. It removes light hydrocarbons (e.g., methane, ethane) from the reformate to produce a stable, high-octane gasoline blending component. These columns are usually made from carbon steel or stainless steel, depending on the presence of corrosive contaminants in the service.
- Recycle Gas Compressor:A compressor is used to recycle hydrogen gas back into the reactors and ensures a continuous supply of hydrogen, which is crucial for maintaining the catalytic reactions. There compressors are typically constructed from materials that can withstand high pressures and hydrogen environments, such as stainless steel or high-strength alloys.
- Catalyst Regeneration System: This system is used to regenerate the catalyst by burning off coke deposits. It restores the activity of the catalyst, allowing for continuous or semi-continuous operation of the unit and is usually made from high-temperature alloys to withstand the regeneration conditions.
- Feed Surge Drum: Its function is to accumulate and stabilize the feedstock before it enters the reactors. It ensures a steady flow of feedstock into the reactors, preventing fluctuations that could affect the reforming process. Surge drums are usually constructed from carbon steel or low-alloy steel.
- Effluent Coolers: Some HEs are used to cool the reactor effluent before it enters the separator, so the cooler reduces the temperature of the effluent to facilitate the separation of hydrogen and hydrocarbons. These coolers are often made from carbon steel or stainless steel.
- Chloride Guard Beds: These are vessels containing adsorbents to remove chlorides from the recycle hydrogen stream. They protect the catalyst from chloride poisoning, which can deactivate the catalyst, and are typically constructed from stainless steel or other corrosion-resistant materials.
These components work together to ensure the efficient and continuous operation of the catalytic reforming process, producing high-octane gasoline and valuable hydrogen byproducts. Proper material selection and maintenance are crucial to prevent corrosion and other forms of degradation in these high-temperature, high-pressure environments.
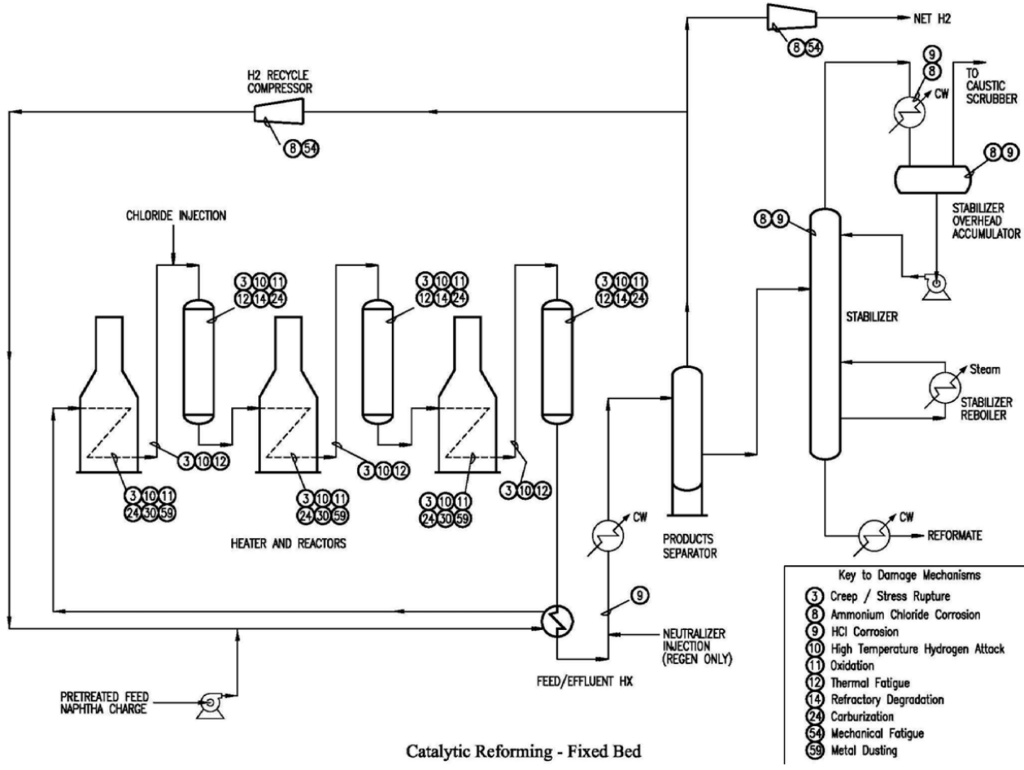
Primary Damage Mechanisms in a Catalytic Reformer Unit
CRUs are subject to various damage mechanisms due to the high temperatures, pressures, and reactive environments involved in the process. The following schematic shows the primary damage mechanisms governing the main pieces of equipment in a CRU.
- High-Temperature Hydrogen Attack (HTHA)
- Description: Occurs when hydrogen reacts with carbides in steel to form methane, leading to the formation of bubbles, cavities, and fissures
- Affected Materials: Carbon steel and low alloy steels
- Critical Factors: High temperatures and hydrogen partial pressures above the Nelson Curve in API 941
- NDT Methods: Ultrasonic testing (UT), especially time-of-flight diffraction (TOFD), and acoustic emission testing (AET)
- Carburization
- Description: Absorption of carbon into the material at elevated temperatures, leading to the formation of metal carbides and loss of ductility
- Affected Materials: Carbon steel, low-alloy steels, stainless steels, and nickel-based alloys
- Critical Factors: High gas phase carbon activity and low oxygen potential
- NDT Methods: Metallography, hardness testing, and field metallography and replication (FMR).
- Metal Dusting
- Description: Severe form of carburization in which carbon deposits lead to the disintegration of the metal into a dust-like form
- Affected Materials: Low-alloy steels, stainless steels, and high-alloy steels
- Critical Factors: Carburizing environments and high temperatures
- NDT Methods: Visual inspection, UT, and RT
- Sulfidation
- Description: Corrosion due to the reaction of sulfur compounds with the metal, forming metal sulfides
- Affected Materials: Carbon steel, low-alloy steels, and stainless steels
- Critical Factors: Presence of sulfur compounds and high temperatures
- NDT Methods: UT measurements and visual inspection
- Chloride Stress Corrosion Cracking (ClSCC)
- Description: Cracking due to the combined effects of tensile stress and a corrosive environment containing chlorides
- Affected Materials: Austenitic stainless steels
- Critical Factors: Presence of chlorides, tensile stress, and temperatures typically above 140°F
- NDT Methods: Wet fluorescent magnetic particle testing (WFMT), UT, and RT
- Ammonium Chloride Corrosion
- Description: Corrosion due to the formation of ammonium chloride salts, which can absorb water and form hydrochloric acid
- Affected Materials: Carbon steel and low-alloy steels
- Critical Factors: Presence of ammonia and chlorides, and condensation of water
- NDT Methods: Visual inspection, UT, and corrosion probes
- Hydrochloric Acid (HCl) Corrosion
- Description: General and localized corrosion due to the presence of hydrochloric acid, which is highly aggressive to most common materials
- Affected Materials: Carbon steel, low-alloy steels, and stainless steels
- Critical Factors: HCl concentration, temperature, and presence of water
- NDT Methods: UT, RT, and visual inspection
- Creep/Stress Rupture
- Description: Time-dependent deformation and eventual rupture of materials under constant stress at high temperatures
- Affected Materials: High-temperature alloys, including Cr-Mo steels
- Critical Factors: High temperatures and sustained stress
- NDT Methods: UT, RT, and metallography
- Reheat Cracking
- Description: Cracking that occurs during the reheating of welds, typically in Cr-Mo steels
- Affected Materials: Cr-Mo steels
- Critical Factors: High temperatures and welding residual stresses
- NDT Methods: UT, RT, and visual inspection
- Corrosion Under Insulation (CUI)
- Description: Corrosion occurring under insulation due to water ingress, often exacerbated by the presence of chlorides
- Affected Materials: Carbon steel and low-alloy steels
- Critical Factors: Presence of water and insulation materials
- NDT Methods: Visual inspection, UT, and RT
- Wet H2S Damage
- Description: Corrosion and cracking due to the presence of hydrogen sulfide in wet conditions
- Affected Materials: Carbon steel and low-alloy steels
- Critical Factors: Presence of H2S, water, and tensile stress
- NDT Methods: UT, magnetic particle testing (MPT), and visual inspection
- Oxidation
- Description: Form of corrosion that occurs when metals react with oxygen at elevated temperatures, forming oxides on the metal surface; this can lead to the thinning of the metal and eventual failure if the oxide layer is not protective or if it spalls off
- Affected Materials: Carbon steel, low-alloy steels, stainless steels, and high-alloy steels
- Critical Factors: Temperature, alloy composition, oxygen availability, exposure time
- NDT Methods: Visual inspection, UT, RT, FMR, hardness testing, magnetic permeability testing
- Thermal Fatigue
- Description: Occurs due to cyclic thermal stresses resulting from repeated heating and cooling cycles that cause expansion and contraction of materials, leading to the initiation and propagation of cracks over time; particularly prevalent in areas where there are significant temperature gradients or where rapid temperature changes occur
- Affected Materials: Carbon steel, low alloy steels, stainless steels, and high alloy steels
- Critical Factors: Temperature cycles, thermal gradients, material properties, design and geometry, operational practices (rapid heating and cooling during start-ups, shutdowns).
- NDT Methods: Visual inspection, UT, TOFD, RT, dye penetrant testing (DPT), MPT, thermography
- Refractory Degradation
- Description: Occurs due to various forms of mechanical damage (cracking, spalling, and erosion) as well as corrosion due to oxidation, sulfidation, and other high-temperature mechanisms; the degradation can lead to loss of insulating properties, exposure of the underlying metal to high temperatures, and potential failure of the refractory lining
- Affected Materials: Insulating ceramic fibers, castables, refractory brick, plastic refractories
- Critical Factors: Refractory selection, design, and installation; thermal shock and thermal expansion; dry out schedules and cure times; anchor materials; erosion and abrasion resistance; process environment
- NDT Methods: Visual inspection, infrared (IR) thermography, UT, AET, hardness testing
These damage mechanisms highlight the importance of selecting appropriate materials and implementing effective inspection and monitoring strategies to ensure CRU integrity and reliability.y
Industrial CRU Lessons Learned
This section highlights several past industrial experiences from which key lessons have been drawn. These insights should be considered and incorporated, as appropriate, into the operation, inspection, or maintenance of existing CRUs.
1. Flange Leaks in High-Pressure Hydrogen Piping
Numerous flange leaks occurred in high-pressure hydrogen piping, which was subject to severe cyclic service. These leaks were attributed to several factors, including incorrect hanger placement, rough flange finishes, improper flange bolting and torquing, and the use of plate-type flanges. By ensuring that flange faces were machined square with a gasket surface finish of 125 AARH or better, and by using spiral wound gaskets with both inner and outer rings, a significant reduction of approximately 90% in flange leaks was achieved. Flange bolting was tensioned by a specialized contractor to ensure optimal bolt loading for maintaining a seal. In the high-temperature sections of the system, bolting was inspected to confirm that grade B-6 bolts were used in services exceeding 800°F to minimize bolt relaxation. Additionally, based on a limited piping stress analysis, piping flexibility was enhanced where necessary to reduce flange bending and loads. Plate flanges were removed and replaced with standard weld-neck flanges.
2. Cracking Reactor Feed and Effluent Piping
A CRU plant experienced a leak in the 26-inch diameter 1.25Cr-0.5Mo steel inlet line to Reactor “C.” The leak was located at a crack adjacent to a long seam weld, measuring 2 inches on the outer diameter (OD) but 5 feet on the inner diameter (ID). The crack was caused by creep; however, metallurgical evaluation confirmed that the tensile and creep properties of both the weld and base material were essentially “like new” ASTM A-387, Grade 11 material.
The occurrence of creep was attributed to increased stress concentrations at the long seam, resulting from welding defects such as improper fit-up (high-low alignment) of the mating sides of the rolled and welded plates and OD-to-ID weld offset. Additionally, the type of welding flux used during the welding of the long seams on the ID of the pipe was believed to have contributed to the initiation of creep cracks.
The unit was taken offline for inspection and repairs for approximately two months. All long seam welds in the reactor inlet and outlet piping were inspected using RT and UT. One additional area of similar long seam creep cracking was detected in the inlet piping to Reactor “E.” The cracked piping sections were replaced later.
3. Downstream Corrosion Due to High Chloride Levels in Make Gas
Corrosion issues have been reported at mixing points or in downstream units when the make gas exported from the CRU contained high levels of chlorides. In one refinery, a leak developed at a piping weld downstream of the interstage condenser at the hydrogen booster compressors. The CRU make gas was mixed with hydrogen plant gas just before entering these booster compressors. The corrosion occurred where the compressed hydrogen was believed to be wet, leading to very localized corrosion of the weld, particularly in the heat-affected zone (HAZ), which created a narrow circumferential groove at the weld fusion line. This groove was not detected by UT but was clearly visible on RT film.
A similar failure occurred in another plant at a tee where cat reformer hydrogen mixed with hydrogen plant hydrogen. High chloride levels in the make gas, resulting from deferred replacement of spent adsorbent in the chloride adsorbers, caused ClSCC in one of the towers, leading to $1 million in retubing costs.
4. Ammonium Chloride Fouling of Feed/Effluent Exchangers
Upsets in upstream hydrotreating units that permit high nitrogen levels in naphtha feed have resulted in rapid fouling of the effluent side of feed/effluent exchangers and associated downstream equipment by ammonium chloride salts.
5. HTHA of C-0.5Mo Steel Feed/Effluent Exchangers
One refinery has found HTHA damage in various C-0.5Mo steel components in feed/effluent exchangers. This damage was believed to have been caused by unknowingly operating slightly above the Nelson Curve limits. There was a lack of actual temperature measurements within the bank of multiple exchangers. Temperature profiles were estimated from heat transfer models, but some component parts of exchangers (i.e., tailpiece, dead areas of shell around floating head, etc.) were difficult to estimate.
6. 885°F (473◦C) Embrittlement of Type 405 SS Reactor
A Type 405 stainless steel (containing 14.3% Cr) in a reactor failed in a brittle manner due to 885°F (473◦C) embrittlement. It had been in service for a long time at about 900°F (482◦C). Type 410 stainless steel, with lower chromium level, should be used in this service, but it requires PWHT.
7. Wet H2S Cracking of Stabilizer Column
A stabilizer experienced significant wet H₂S cracking at the tray ring support attachment welds in the upper section of the column. While this cracking is not expected to occur during normal operation—due to the absence of free water and very low levels of H₂S—monitoring has revealed that the cracks have been actively growing over the years. It is believed that the cracking occurs during periods of column water washing, which is done to remove ammonium chloride deposits from the upper section of the column.
8. Ammonium Chloride Fouling of Stabilizer Tops
Upsets in upstream hydrotreating units that allow high nitrogen levels in naphtha feed have led to rapid fouling of the stabilizer column’s top section and overhead system due to ammonium chloride salts.
Summary
In supporting the safety and productivity of CRUs across the oil and gas and other industries, Equity subject matter experts (SMEs) can develop and/or review Corrosion Control Documents (CCDs), Damage Mechanism Reviews (DMRs), and Integrity Operating Windows (IOWs). These analyses and deliverables offer in-depth information and recommendations regarding the specific damage mechanisms to which any particular equipment may be susceptible. Reach out to us to learn more about how our team can help anticipate and address damage mechanisms and ensure your CRUs run more reliably and efficiently.
Please submit the form below with any questions for the author:
References:
- API RP 571 – Part 1
- NACE Corrosion Control in the Refining Industry – Part 1
- WRC_489_v22.pdf – Part 1
- WRC_490_v10.pdf – Part 1
- WRC_489_v22.pdf – Part 2
- API RP 941 – Steels for Hydrogen Service at Elevated Temperatures