Amine treating units are important components in midstream processing, as they are responsible for removing acid gases such as CO₂ and H₂S from hydrocarbon streams. However, these units pose significant corrosion and cracking challenges which account for many midstream reliability issues. This article provides a comprehensive overview of the amine treating process, discusses rich/lean amine loading, identifies key damage mechanisms and areas of concern, and offers recommendations for corrosion mitigation. Additionally, it outlines the importance of applying a risk-based inspection (RBI) methodology to the integrity management of these units.
Amine Unit Process Overview
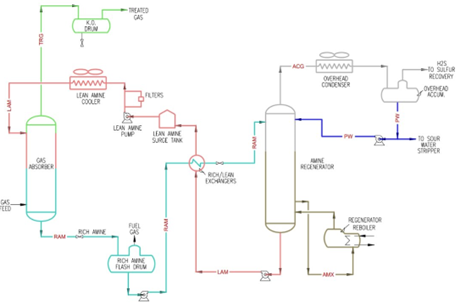
Amine treating units utilize a lean amine solution to strip CO₂ and/or H₂S from the inlet feed of the absorber. The process begins with the sour gas entering the absorber, where it contacts the downflowing lean amine solution. This interaction facilitates the absorption of acid gases, resulting in a treated hydrocarbon feed overhead and a rich amine containing CO₂ and/or H₂S at the bottom. The treated gas, now free of most acid gases, is sent to downstream processes or storage, while the rich amine is routed to a regenerator (or still). In the amine regenerator/still, the rich amine enters at a high temperature and low pressure to strip off the absorbed gases. This process occurs in a distillation column where the amine solution is essentially boiled and the acid gases are driven off as vapors. The overhead acid gas vapors, primarily consisting of CO₂ and/or H₂S (along with entrained water), are condensed and separated. The regenerated lean amine, now free of acid gases, is cooled and recycled back to the absorber to continue the gas treating cycle. Both lean and rich amines present unique challenges throughout different areas of the unit, necessitating careful monitoring and maintenance.
Amine Loading and Heat Stable Amine Salts (HSAS)
Amine Loading
Amine loading refers to the concentration of acid gases, such as H₂S and CO₂, absorbed by the amine solution. This parameter is critical in determining the efficiency and safety of the amine treating process. Rich amine loading, which indicates the amount of acid gas absorbed by the amine solution after it has contacted the sour gas, typically ranges from 0.3 to 0.5 mol/mol. On the other hand, lean amine loading, which represents the acid gas concentration in the amine solution after regeneration, is generally maintained between 0.005 and 0.02 mol/mol. Improper amine loading can lead to significant operational issues and increased corrosion in both rich and lean handling components. For instance, overstripping of lean amine, which involves removing too much acid gas during regeneration, can result in higher rich amine loading. This condition increases the risk of corrosion, particularly in carbon steel equipment, due to the higher concentration of acid gases in the rich amine. Conversely, understripping (i.e., where insufficient acid gas is removed) can reduce the capacity of the lean amine to absorb additional acid gases, leading to inefficient operation and potential process upsets. Maintaining proper amine loading is crucial to effective unit operation and corrosion control. It ensures that the amine solution has the optimal capacity to absorb acid gases while minimizing the risk of corrosion.
Heat Stable Amine Salts (HSAS)
HSAS form when amines react with contaminants other than CO₂ and H₂S, such as oxygen, chlorides, and sulfates. These salts are highly corrosive and can significantly affect both rich and lean amine systems. Maintaining HSAS levels below 2 wt% is crucial to prevent excessive corrosion. The presence of excessive HSAS can reduce the amount of active amine available for acid gas absorption, leading to higher rich amine loading and increased corrosion rates. Regular monitoring and control of HSAS levels are essential to ensuring the longevity and reliability of amine treating units.
Key Damage Mechanisms and Areas of Concern
Amine Corrosion
Amine corrosion is not caused by the amine molecule itself; rather, it is influenced by dissolved CO₂ and H₂S, HSAS, and other degradation products. The severity of corrosion increases with temperature and is generally most aggressive in MEA amine solutions, followed by DGA, DIPA, DEA, and MDEA. Amine corrosion is most prevalent in hot rich amine piping and equipment, in addition to the regenerator/still reboiler where the temperature and turbulence of the amine stream are the highest. Proper operation of the amine system – with particular attention to acid gas loading levels and the buildup of HSAS – is the most effective method of corrosion control.
CO₂ Corrosion
Carbon dioxide in the presence of liquid water forms carbonic acid, which is corrosive to carbon steel. Because amine solution contains water which can react with the CO₂ and thus lead to carbonic acid attack, CO₂ corrosion is a significant concern in amine units that treat for CO₂ acid gases. The partial pressure of CO₂ plays a critical role, with pressures above 4 psia being particularly concerning for localized corrosion. This type of corrosion is most prevalent in rich amine piping/equipment and regenerator/still overhead systems where CO₂ concentrations may be high.
Amine Stress Corrosion Cracking (SCC)
Amine stress corrosion cracking (SCC) is another issue in lean amine handling systems, particularly in non-post-weld heat-treated (non-PWHT) carbon steel equipment. This form of alkaline SCC occurs when carbon steel components are exposed to amine solutions, especially at elevated temperatures. The susceptibility of carbon steel to amine SCC is influenced by several factors, including the type of amine used, the operating temperature, and the presence of residual stresses. The stress needed to initiate and propagate a crack in carbon steel can arise from the applied pressure of the operating system or from residual stresses introduced during the welding process. These residual stresses are often concentrated in the weld metal and the heat-affected zones (HAZs) of welded joints. The presence of these stresses, combined with the corrosive environment of the amine solution, can lead to the initiation and propagation of cracks. PWHT is the most common and effective method to reduce the likelihood of amine SCC.
Wet H₂S Cracking
The presence of H₂S in combination with water facilitates the generation of atomic hydrogen on the steel surface through a corrosion reaction. This atomic hydrogen then diffuses into the steel, where it can accumulate at inclusions, laminations, or other discontinuities, thus leading to various forms of damage. H₂S cracking is commonly found in rich amine systems, particularly in non-PWHT carbon steel equipment. The rich amine solution, which is abundant with absorbed H₂S, creates an environment conducive to several types of hydrogen-related damage mechanisms. These include hydrogen blistering, hydrogen-induced cracking (HIC), stress-oriented hydrogen-induced cracking (SOHIC), and sulfide stress cracking (SSC).
Erosion-Corrosion
High process stream velocities can cause localized thickness loss in amine service. Erosion-corrosion is a concern in areas with high flow rates, such as pump discharge lines and control valve outlets. The regenerator/still reboiler may also contain turbulent areas of flow where erosion-corrosion will occur. The maximum recommended velocities are approximately 5 fps for rich amine and 20 fps for lean amine. Proper design and material selection, along with regular monitoring, can help mitigate this issue.
Ammonium Bisulfide Corrosion
Amine degradation products may react with H₂S to form ammonium bisulfide, causing localized corrosion; this is a particular concern in the top of the still tower and acid gas overhead condenser. This type of corrosion is influenced by the concentration of ammonium bisulfide and flow dynamics within the system. This mechanism is only a concern in H₂S removal units where ammonia is potentially present. Ensuring an effective purge in regenerator overhead systems is key to limiting ammonium bisulfide concentrations and corrosion.
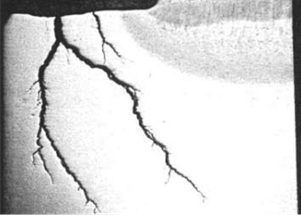
Figure 2: Amine SCC on Non-PWHT CS

Figure 3: Erosion-Corrosion on Reboiler Return Elbow
Recommendations/Mitigation
Material Selection and Treatment
Using PWHT carbon steel for piping and equipment can significantly reduce susceptibility to amine SCC and wet H₂S cracking. This treatment reduces the hardness of the weld and HAZ, thereby lowering the risk of SCC and hydrogen-induced cracking (HIC). In areas with high corrosion potential, such as the reboiler outlet, upgrading to stainless steel can provide additional protection. Stainless steels, particularly 316L, are preferred for their improved resistance to amine corrosion over carbon steel (especially at higher temperatures). Additionally, the use of stainless steel can mitigate the risk of amine SCC, most notably in higher-temperature, lean amine handling piping/equipment.
Operational Controls
Maintaining proper amine loading is essential to the prevention of overstripping or understripping, both of which can lead to operational inefficiencies and increased corrosion rates. As mentioned, overstripping can increase rich amine loading, leading to higher corrosion rates in the rich amine piping and equipment. Conversely, understripping can lead to lower-than-ideal rich amine loading and cause potential plant upsets and/or hydrocarbon product not meeting proper specs. Regularly monitoring and controlling HSAS levels are crucial to prevent excessive corrosion within the unit. Implementing nitrogen purging on lean amine surge tanks can prevent oxygen ingress, which contributes to the formation of HSAS. Proper temperature and flow control are also critical, as high temperatures and turbulent flow can lead to increased corrosion. Additionally, regular filtration and monitoring of amine solids content can help manage the buildup of FeS corrosion products that can otherwise lead to unit fouling and increased corrosion.
Inspection and Monitoring
Conducting regular amine sampling to track amine strength, composition, and HSAS levels is crucial for maintaining the integrity of the amine system. Additionally, variables such as pH, iron, formate, acetate, and bicine are important to screen for during lean amine sampling. Amine strength and composition can affect the solution’s ability to absorb acid gases and its corrosivity. Utilizing advanced inspection techniques such as ultrasonic testing (UT) and radiographic testing (RT) can help detect localized corrosion and cracking. UT is effective for measuring wall thickness and detecting internal flaws, while RT can provide images of the internal structure of piping and equipment, revealing cracks and other defects. Other techniques such as phased array ultrasonic testing (PAUT) and/or wet fluorescent magnetic particle testing (WFMT) may help identify cracking indications from either amine SCC or wet H₂S cracking.
Risk-Based Inspection (RBI)
RBI is a dynamic methodology that estimates current and projected future risk evaluations based on data and knowledge at the time of the assessment. RBI uses both the probability of failure (POF) and the consequence of failure (COF) to prioritize and optimize inspection and maintenance activities. The POF is influenced by factors such as age, material properties, operating conditions, presence of corrosive species, and time since the most recent inspection, while the COF considers the potential impact of a failure on safety, environment, and business operations.
The calculated COF does not change over time and is constant throughout the given vessel/piping circuit. However, POF increases over time as the vessel/piping circuit ages and becomes more damaged due to corrosion. Completing highly effective inspections specific to the damage type identified by a trained materials and corrosion engineer allows for a better understanding of the damage state present in the given vessel/piping circuit. Inspection effectiveness is directly related to the inspection technique used and percentage of the identified susceptible area examined.
Regular reassessments are necessary to incorporate new inspection data, changes in process conditions, and the effectiveness of mitigation strategies. This ensures that the RBI program remains relevant and effective in managing the risks associated with the amine system. By continuously updating the RBI assessment, operators can make informed decisions on inspection intervals, maintenance activities, and material upgrades to enhance the reliability and safety of the system.
In summary, a comprehensive approach that includes proper material selection and treatment, operational controls, regular inspection and monitoring, and a robust RBI program is essential for managing the risks associated with amine systems. This approach helps to prevent corrosion-related failures and ensure the long-term reliability and safety of the equipment.
Please submit the form below with any questions for the authors:
Further reading:
- API RP 571, “Damage Mechanisms Affecting Fixed Equipment in the Refining Industry” 3d Ed., 2020.
- API RP 945, “Avoiding Environmental Cracking in Amine Units” 4th Ed., 2022.
- WRC Bulletin 489 – 2nd Edition: Damage Mechanisms Affecting Fixed Equipment in the Refining Industry, Part 1. Welding Research Council, Inc., 2004.
- API RP 581, “Risk-Based Inspection Methodology” 3d Ed., 2016.